FDA Deputy Commissioner for Foods and Veterinary Medicine, Dr. Stephen Ostroff (pictured above), presented on Food Fraud at the October 30-31, 2017 “FOOD FRAUD – GLOBAL UNDERSTANDING: Global Action for Prevention and Mitigation of Food Fraud” conference here in Beijing. Since his presentation last April there are several new slides that provide more insight and detail – the text is in the report here; screenshots of the slides are also included.
Summary: FDA has been consistent in its direction and activities – all types of Food Fraud have been illegal since the adoption of the Food Drug & Cosmetics Act of 1938 (FDCA or FD&C). The Food Safety Modernization Act (FSMA) just builds upon that Act and strengthens the agency enforcement. The recent update from FDA reinforces that:
- All types of Food Fraud are illegal.
- To determine “hazards that require a preventive control”, an assessment for all types of fraud is needed.
- Addressing Food Fraud is NOT covered in the FDA Food Defense plans.
The publicly and formally presented FDA updated slides and comments include:
____________
- FDA using the Food Fraud term for Economically Motivated Adulteration (EMA): It may be specifically for this “Food Fraud”titled conference, but FDA has continued to use the term “Food Fraud” when explaining how to address the previously defined FDA term of “economically motivated adulteration.”
Title of FDA Presentation:
“Towards Global Action for Prevention and Mitigation of Food Fraud,” Deputy Commissioner for Foods and Veterinary Medicine (responsible for all food), Dr. Stephen Ostroff, MD.
____________
- Economically Motivated Adulteration: FDA acknowledgement of the global nature of the problem and also concerns beyond public health to economic loss and lower consumer confidence.
Slide Title: Economically Motivated Adulteration A problem that occurs throughout the world
- Developed country setting and less developed country settings
- It’s something that unfortunately binds all of us together
- Economic & Law Enforcement importance
- Public health importance
- Food safety
- Loss of confidence in food supply
____________
- EMA- Consumer Perception: FDA further defined the concerns.
Slide Title: EMA – Consumer Perception
- Significant consumer anger at fraudulent food products and dietary supplements
- Whether food quality or food safety issue
- Resulting industry harm/reputation
- Social media
- Consumers vote with their wallet and purse
- Potential loss of confidence in integrity of food supply

____________
- Legality: Dr. Ostroff emphatically and clearly reiterated that Food Fraud has been illegal since at least 1938 – and for all types of fraud (from adulterant-substances to stolen goods and counterfeits) and all types of products (from ingredients to packaged goods).
Slide Title: Legality
“All types of food fraud have been illegal in the U.S. since the adoption of the Federal Food, Drug, and Cosmetics Act of 1938.”

____________
- Why EMA is not in the “Intentional Adulteration” Rule: There is continued confusion about the IA rule: (1) the phrase “intentional adulteration” covers all types of intentional illegal acts but for FDA it does not, and (2) that intentional EMA/food fraud is covered in the “preventive controls” rule.
Slide Title: Why Isn’t Economically Motivated Adulteration Covered by the Intentional Adulteration Rule?
The goal of the final rule on intentional adulteration is to prevent acts intended to cause wide-scale harm to public health, including acts of terrorism targeting the food supply.

____________
- FSMA-PC General Requirements: While FSMA only requires preventive controls for public health threats, there must be an assessment to define what is (or isn’t!!!) a hazard.
TO REINFORCE: A vulnerability assessment for all products must be conducted to define what is NOT a “hazard that requires a preventive control.” If there is an investigation after a Food Fraud incident there would be a question of “how did you determine this to NOT be a hazard that requires a preventive control?” While this may seem like a major challenge, the basic requirements are not very complex: (1) GFSI already requires a Food Fraud Vulnerability Assessment for all types of fraud and all products, and (2) all vulnerabilities are not risks and all risks are NOT hazards that require preventive controls.
Slide Title: Requirements Included Under Preventive Controls Rules
- Develop food safety plan
- Conduct risk assessment/ hazard analysis
- Develop risk-based preventive controls to mitigate hazards
- Verify that preventive controls are working
- Economically motivated adulteration that is potentially dangerous to human or animal health included

____________
- EMA/FF Hazards to Include: FDA reiterated their inspection and enforcement priority on public health harms
Slide Title: Hazards to Include
- Hazards that may be intentionally introduced for economic gain will need preventive controls in rare circumstances, usually where there has been a pattern of such adulteration
- These hazards are “reasonably foreseeable”
- Such hazards can emerge in both food for humans and animals
- FDA encourages facilities to adopt other measures to address the risks of economically motivated adulteration not covered by preventive controls rules

____________
- FSMA-FSVP: Anyone exporting to the US must comply with the same rules, and that the FDCA is also still the rule of the land.
Slide Title: Foreign Supplier Verification System
- Under the final rule on foreign supplier verification systems, hazards again are only relevant if they have the potential to cause illness or injury
- An importer’s hazard analysis must identify “reasonably foreseeable hazards” intentionally introduced for purposes of economic gain

____________
Food Fraud is being more formally addressed as a regulatory issue around the world and by FDA. FDA continues to provide clarification on addressing Food Fraud. FDA has been consistent in their message, essentially since 1938. FDA has also been clear that their priority will be on public health hazards while emphasizing that all types of Food Fraud are illegal, unfit for commerce, and could lead to a recall.
Reference: Ostroff, Stephen (2017). Towards Global Action for Prevention and Mitigation of Food Fraud, FOOD FRAUD – GLOBAL UNDERSTANDING: Towards Global Action for Prevention and Mitigation of Food Fraud, Beijing, China, October 30, 2017, Quebec City, October 30l 5, 2017), http://foodfraudbeijing.medmeeting.org/Useren/login/6509
Blog Post: Review of FDA Presentation on Food Fraud and Economically Motivated Adulteration – FDA Deputy Commissioner for Foods Dr. Stephen Ostroff
by John Spink • May 31, 2017 • Blog •
FDA Deputy Commissioner for Foods and Veterinary Medicine, Dr. Stephen Ostroff, presented on Food Fraud at the April 4-5, 2017 Food Fraud Conference, Quebec City . FDA has been consistent in its direction and activities – all types of Food Fraud has been illegal since the adoption of the Food Drug & Cosmetics Act of 1938. FSMA just builds upon that Act and strengthens the agency enforcement.
FDA Presentation on Food Fraud
FDA Deputy Commissioner Ostroff opened the regulatory session where he defined the terms, discussed the scope, and provided a realistic perspective on the priority-setting by FDA.
This presentation was significant since it appears to be the first time that an FDA presentation holistically addressed or mentioned Food Fraud. Before this conference a “Food Fraud” keyword search on FDA.gov had nine results which included comments from the FDA EMA meeting by USP (1) and Food Fraud Prevention Academy (FFPA) (3), a reference to the USP Food Fraud Database (3), a comment in the FSMA Intentional Adulteration final rule (1), and in a transcript of a public meeting (1).
Review of Dr. Ostroff’s presentation contents and slides (Note: includes ADA-compliant descriptions of the figures):
- Food Fraud Risk Matrix: To define the terms, Dr. Ostroff used the Food Risk Matrix to explain the different types of food risks. The slide cited “Adapted from: Spink (2006), The Counterfeit Food and Beverage Threat, Association of Food and Drug Officials (AFDO), Annual Conference 2006.” He commented on the efficiency of separating the risks but also the challenge of the prevention versus the objectives or statutory boundaries of agencies. (Figure 1. The Food Continuum — citing Spink 2006, a presentation of the food risk matrix including food quality, food safety, food fraud and food defense.)

- FDA Working Definition of Economically Motivated Adulteration: To review the base concepts and boundaries he reviewed the FDA working definition that was originally in a Federal Register published meeting invitation for the FDA-wide public meeting on Economically Motivated Adulteration. His emphasis was on “intentional,” “a substance”, and “for economic gain.” [NOTE: This working definition has not been updated and it is not explicitly cited in the FSMA law, regulations, or guidance documents. The Qualified Individual training documents use terms such as EMA, economically motivated hazard, and economically motivated food safety hazard.] (Figure 2. How does FDA Think of Economically Motivated Adulteration (EMA) – quoted the FDA working definition of EMA from the Federal Register published meeting invitation.)

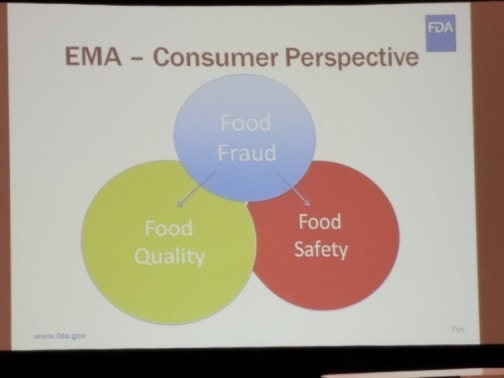
- Consumer Perspective – All Issues: He noted that consumers were focused on both the Food Quality and Food Safety aspects of Food Fraud. (Figure 3. EMA- Consumer Perspective – a ven diagram demonstrating consumer concern of food fraud in terms of equal focus on food quality and food safety.)
- Consumer Perception: He noted there is “significant consumer anger” for a range of consumer issues, from being cheated to food safety concerns. The consumer concerns can lead to “industry harm/ reputation.” [NOTE: as with comments from the other countries including China, the consumer concern can expand to loss of confidence in the food supply chain and government overseers.] (Figure 4. EMA- Consumer Perception – includes bulleted list of concerns.)

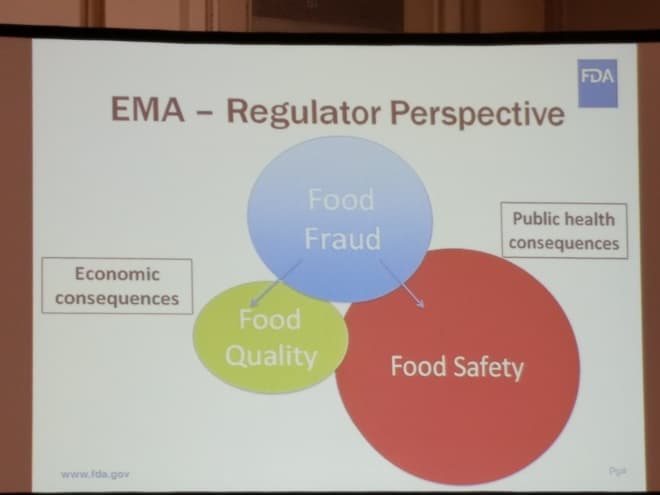
- Regulator Perspective: The FDA regulatory focus is on health hazards and specifically ‘hazards that require a preventive control.’ (Figure 5. EMA – Regulator Perspective – referring to the consumer perspective in Figure 3, this is a ven diagram demonstrating [food] regulator concern of food fraud in terms of low focus on food quality and 4x greater concern with food safety incidents.)
- Government Priority Setting: “In circumstances where no regulatory agency has unlimited resources, where and how does EMA [Food Fraud] fit onto a list of priorities?” This statement was a starting point for describing the FDA scope and focus. (Figure 6. Quote – content noted above)

- Regulator Activity – FSMA and FDCA: He noted the details of the Food Safety Modernization Act (FSMA) and also emphasized that the Food Drug & Cosmetics Act (FDCA or FD&C Act) has been in place since 1938. All types of Food Fraud have been illegal regardless of the actual health hazard.
From his slide on “Regulator Response” he reiterated sections of FDCA that clarified all types of fraud – for both raw materials and finished goods – are illegal, unfit for commerce, and subject to a recall. [NOTE: Knowledge of illegal products allowed to enter commerce is a crime.]:
- “Adulterated foods
- Section 402 (a) of the FD&C Act [FDCA] (21 USC 342): “If it bears or contains any poisonous or deleterious substance which may render it injurious to health.
- Section 402 (b): “if any valuable constituent has been in whole or in part omitted or abstracted therefrom; or if any substances has been substituted wholly or in part…; or if damage or inferiority has been concealed… or if any substance has been added thereto or mixed or packed therewith, so as to increase its bulk or weight, or reduce its quality or strength, or make it appear better or of greater value than it is.”
- “Misbranding
- Section 403(b) of the FD&C Act (21 USC 343 (B)): “offered for sale under the name of another food.”
- Section 403(a): “labelling is false or misleading”
- Section 403(i): ingredient labeling”
- Strategy for Identifying the “Next Melamine”: He addressed the ultimate goal, which is to predict the next Food Fraud incident… or to identify “the next Melamine.” Very broadly he presented the Criminology and Situation Crime Prevention concepts where the expected reward is greater than the expected risk or penalty. This is the starting point to prioritize prevention countermeasures. (Figure 7. Strategy for Identifying the ‘Next Melamine’ – content noted above.)

- EMA Risk Factors: Specifically, he expanded the risk factors to include: inherent vulnerabilities, difficulties in detection (both “inadequate to detect” and “inappropriate methods may give spurious evidence”), supply disruptions increase arbitrage benefits, and the expanding fraud opportunity based on globalization (complex supply chains with products that are moving farther and faster around the world). (Figure 8. EMA Risk Factors & Figure 9. (Continued) – content noted above.)


- Recommendations by the US Government Accountability Office (2011): He reviewed how FDA responded to the GAO recommendations by: adopting a working definition (uses the Federal Register published definition from the meeting invitation), providing written direction on how the FDA centers should address the issue, and then increasing communication. (Figure 10. Review of GAO report on EMA – content noted above.)

- GAO Recommendation – Workgroup on Economically Motivated Adulteration (WEMA): He noted that this group was created in response to the GAO report. The group is ongoing. (Figure 11. Workgroup on Economically Motivated Adulteration (WEMA) – summary of activities.)

- FDA Focus on Collaboration: The FDA focus for addressing this issue is to collaborate not only within the US but also globally. [FF NOTE: Countries around the world are challenged by the interdisciplinary and international nature of the fraud and that the prevention is fundamentally different than for the traditional food safety hazards.] (Figure 12. Greater Collaboration is Essential – content noted above.)

- FDA Focus on Collaboration – Role of Industry: The presentation emphasized the importance of industry leadership. He also specifically noted the work of the US Pharmacopeia. The FDA presentation closed with another emphasis that collaboration was key for success. (Figure 13. Role of Industry, and Figure 14. Collaboration Essential for Success – content noted above.)